About Us
We are eager to fight cancer alongside you!
Who We Are
Full-service, oncology-focused clinical trial provider.
Theradex will help you navigate through the challenges of trials for specific target populations. New trial designs and endpoints present new challenges in the conduct of early and later phase oncology trials.
Theradex Oncology is a full-service, oncology-focused clinical trial provider serving the global pharma/biotech industry and the US NCI for more than 40 years. With offices in Princeton, London and Gothenburg, Sweden, we have managed more than 350 sponsor-led trials in North America, Europe and Asia ranging from early to late phase and supported over 2000 trials with our data management, monitoring and auditing expertise developing strong relationships with regulators and sites along the way.
The combination of Medical, Regulatory and Operational Expertise enable Emerging Biotech to thrive by fostering a flexible, nimble structure with our backbone of 40+ years of experience. Theradex Oncology has long term senior managers including US and EU regulatory experts, MD medical oncologists with decades of experience conducting oncology clinical trials and operational teams with an average of 7 years of oncology experience.
For many projects, we guide emerging biotech sponsors through the pre IND and IND submission process and assist for protocol design and development for all clinical phases. Our oncologists work hand-in-hand with the operational team for seamless study execution. Our approach and way of working is collaborative as we navigate regulatory hurdles, overcome complex medical challenges, and execute each study seamlessly as a true partner.


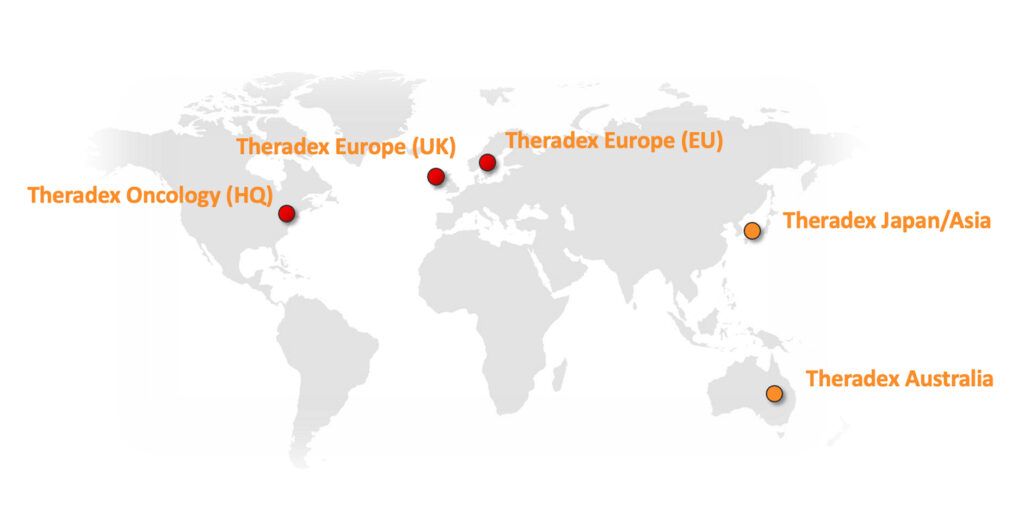
Global Presence
Global Operations & Partnerships
Theradex has been operating in the US, UK and EU for over 25 years. We utilize strategic alliances to expand Theradex’ global presence and have chosen qualified and reputable partners who have agreed to operate to our SOPs and to work under our guidance.
National Cancer Institute
Theradex and the National Cancer Institute (NCI)
For over 40 years, Theradex has played a key role in monitoring early Phase Clinical trials for the NCI. The Clinical Trials Monitoring Services (CTMS) program collects Phase I and II cancer trial data and audits the NCI-supported cancer center programs participating in these early phase trials and national cooperative group clinical trials.
Since 1982, Theradex has held consecutive CTMS contracts and our current agreement extends to May of 2032. This partnership has enabled us to gain significant experience in the treatment of cancer and allied diseases at the premier cancer centers in North America. Through the CTMS, Theradex has also been engaged with the leading clinical investigators involved in early clinical trials during three decades of new anti-cancer therapeutic approaches, from cytotoxic chemotherapy to immunotherapy to targeted therapies requiring genetic profiling of cancer patients.
In March, 2014 the Experimental Therapeutics Clinical Trials Network (ETCTN) was launched by NCI to consolidate and integrate the conduct of all early phase clinical trials across NCI. All ETCTN protocols are monitored through Theradex’s expanding CTMS program.
We share this relationship as we believe that it benefits our sponsors to know of our exposure to novel treatments as well as it has enabled us to create and maintain institutional site and KOL relationships for over 500 studies in the past 10 years.
Indication Experience
- ALL (Ped)
- AML
- MDS
- NSCLC
- MPN
- Bladder
- Breast
- Cervical
- Endometrial
- Esophageal
- Head & Neck
- Glioblastoma
- Melanoma
- Neuroendocrine
- NUT Carcinoma
- Ovarian
- Pancreatic
- Prostate
- Sarcoma
- Solid tumors
- Urothelial
Modalities
Targeted Therapies
- TKIs (e.g. RET, BRAF)
- Other cell signal proteins (e.g. KRAS, AKT)
- DNA Damage/Repair (e.g. TP53, ATR, PARP)
- Endocrine therapies
- Radioligand
Immunotherapies
- ADC
- BITE and Bi-specific Ab
- mAb (e.g. checkpoint inhibitors)
Novel Chemotherapies
- PEGylated
- Liposomal
- Nanoparticle
- Dendrimer Polymers
Cell, Gene and other Therapies
- Oncolytic Virus RNA/DNA
- Bacterial
- Radiosensitizer
- Cell
- siRNA
Cancer Vaccines
- Peptides
- Proteins
- Cell based
- Tissue based
- CPI combination
Countries
- US
- Canada
- UK
- France
- Italy
- Spain
- Germany
- Denmark
- Belgium
- Netherlands
- Australia
- New Zealand
- S. Korea
- Japan
- UAE
- Israel
Everything is working like a clockwork and has been fantastic; an amazing journey, congratulations to everybody, I have run out of words of praising. We have had a few rounds of applause in the in the full company meeting and that definitely includes your work.
